
Feature
The cutting edge of CRISPR is in Nigeria
Just outside the center of Ede, Nigeria, on the campus of Redeemer’s University, a sort of mirage rises from the red earth: a pair of sleek, almost-completed two-story structures surrounded by elegant gardens featuring native plants. The stores along the high road to Ede, population 160,000, are mostly open-faced wooden shelters, their roofs propped up by crooked tree branches. They couldn’t be in sharper contrast to the $11 million headquarters of the African Centre of Excellence for Genomics of Infectious Diseases (ACEGID). Solar panels provide all the power; the gleaming floors are locally quarried granite; the lab is a state-of-the-art biosafety level 3; and the exterior walls are finished with a smooth coat of brown mud—at once modern and proudly African.
ACEGID’s director, Christian Happi, wants his splendid HQ to deliver a bold message: Africa has transcended its colonial past, and African scientists are capable of leading the world. In fact, they already are.

The campus of the African Centre of Excellence for Genomics of Infectious Diseases in Ede, Nigeria
Oladipupo Oguntunde / ACEGID
This year, under Happi’s guidance, ACEGID is ramping up Sentinel, one of the first-ever public health programs to use cutting-edge CRISPR gene editing to surveil for diseases. It’s based on a diagnostic panel called CARMEN, short for Combinatorial Arrayed Reactions for Multiplexed Evaluation of Nucleic acids. Launched in 2020 by the Broad Institute and celebrated in Nature, CARMEN involves extracting viral RNA from patient blood plasma, making copies of it, then dropping these copies onto rubber chips, each one slightly larger than a smartphone and etched with thousands of “microwells.” CARMEN is powered by CRISPR’s most powerful protein, Cas13, which looks for specific viral sequences for a variety of diseases. Happi’s research teams use it to search for 16 blood-borne pathogens, among them Ebola, Lassa fever, West Nile virus, and Mpox.
ACEGID’s research teams collect patient samples at four rural clinics, then transport them to Ede. (It is also testing the technology in Sierra Leone.) The facility aims to ramp up to testing as many as 2,000 by the end of the year—a 12-fold increase, if it works. At that scale, experts believe, health authorities could start to discern where, say, Lassa is hot, or where an outbreak of Mpox is imminent, and whether public warnings and quarantines are needed. Currently, there’s no way to proactively monitor for diseases; local clinics simply alert authorities when they have diagnosed a patient.
Happi has a deep resume. A Harvard postdoc and a professor of molecular biology and genomics at Redeemer’s, in 2014 he diagnosed the first case of Ebola in Nigeria, and in 2020 directed the first sequencing of the SARS-CoV-2 genome by a lab in Africa.
At 56, he is a supremely confident man who still carries a small-town folksiness from growing up in rural Cameroon, where his father drove a bus. If you step into his office, you’re in for a geopolitical sermon. “For decades,” he says when I stop by on a sweltering afternoon in March, “researchers have been taking blood samples back to Europe, back to America. And it doesn’t work. We have to wait months for the results.”
Sign up for Harvard Public Health
Delivered to your inbox weekly.
Even as the Nigerian economy sputters (the inflation rate is currently 34 percent) and as the country’s leaders clamber for foreign investments, Happi has managed to bring in about $100 million in donations over the last five years—from the World Bank, the Elma Foundation and the Skoll Foundation, among others. These days, he juggles research with fundraising for ACEGID, traveling the globe making funding appeals. Whether he’s in London or Paris or Singapore, he wears a shirt in an African print to drive home his principal message: The world needs to shed colonial ideas about epidemiological research in Africa.
Happi is trying to make Africa scientifically self-sufficient. Since ACEGID’s founding in 2014, he’s trained molecular biologists from all 55 African countries except for Eritrea. As we speak, young scientists from Sao Tome and Lesotho are in the next room, learning about genomics. He sees each student as the seed of a revolution, and he sees himself as their stern taskmaster. “If you don’t go back to your country and share what you learned,” he told them earlier that day, “you’ve failed.”
Eventually, Happi tells me, Africa won’t just have a host of qualified scientists. It will, he says, have better laboratories. It will have more factories that make reagents and test tubes. “We shouldn’t have an inferiority complex,” he says. “Let us realize our dreams.”
I look up from my notebook and register what is by far the biggest work of art displayed in the whole room: a photograph that captures an ebullient Barack Obama shaking Happi’s hand.

Christian Happi, photographed at ACEGID on March 5, 2024
Adversity drives Happi’s ambition. When he was eight years old, he got malaria for the umpteenth time. His mother carried him to the local health clinic in Cameroon, and on the way back the pair stopped to rest under a tree. Happi’s siblings had also endured malaria multiple times, and some neighbor kids had died from it. He asked his mom, “Why isn’t there a cure for this disease?” When she said she didn’t know, Happi told her ‘When I grow up, I will find a cure.’”
Arguably, the prediction was a long shot. Happi was the sort of boy who, when sent to the store for milk, would stop at a soccer field, set the milk behind the goal, and play for a couple of hours. He lived amid strict discipline, though. At his Catholic school, he says, the teachers hit kids with sticks for making an error reciting multiplication tables. “And then they sent you home with a note,” he says, “and your parents whipped you again. The beatings worked,” he adds, chortling. “I always got 100 percent.”
During breaks from school, he worked as a laborer. At the local market, he transported bananas and plantains on his head; he carried blocks of concrete at construction sites; he picked cassavas and yams on local farms. In his spare time, he read voraciously, borrowing books from a wealthy friend. He was especially drawn to French comic books and American police stories.
He was less fond of being a postdoc in America. Happi arrived stateside with a PhD in molecular parasitology from the University of Ibadan, in Nigeria. But as he tells it, many of his colleagues regarded him condescendingly and dismissed Africa, saying of its people, “‘You’re not smart enough to make discoveries.’ For me,” he says, “those statements were catalytic.”
In 2011, Happi moved back to Nigeria, resolved to prove he could do world-class science on his home turf. His moment came in 2014, just weeks after he founded ACEGID along with Pardis Sabeti, a computational geneticist at the Broad Institute and a professor of immunology and infectious Diseases at the Harvard T.H. Chan School of Public Health.
One evening that July, while unwinding at home with his young family, he got a call saying a passenger who’d flown into Lagos that day had died with a fever. Could he sequence this man’s blood sample and see if it was Ebola?
Happi’s lab was 90 minutes away, over a rough, unlit road. It was a level 2 biosafety lab, meaning it lacked the high-end equipment required to handle highly lethal specimens such as Ebola, which should be handled in a level 4 context. But Happi thought of the conversation he’d had with his mother long ago, under the tree, about malaria. He remembered the kids who died in his village. He told his wife, Anise, “If I don’t make it back, take care of the children.”
He drove to the lab and worked until dawn to sequence the virus. Then he called Nigeria’s Minister of Health and said, “We have Ebola in the country.” Contract tracing kicked in. As the rest of West Africa endured 11,000 deaths from the virus, Nigeria, population 227 million, lost but eight people.
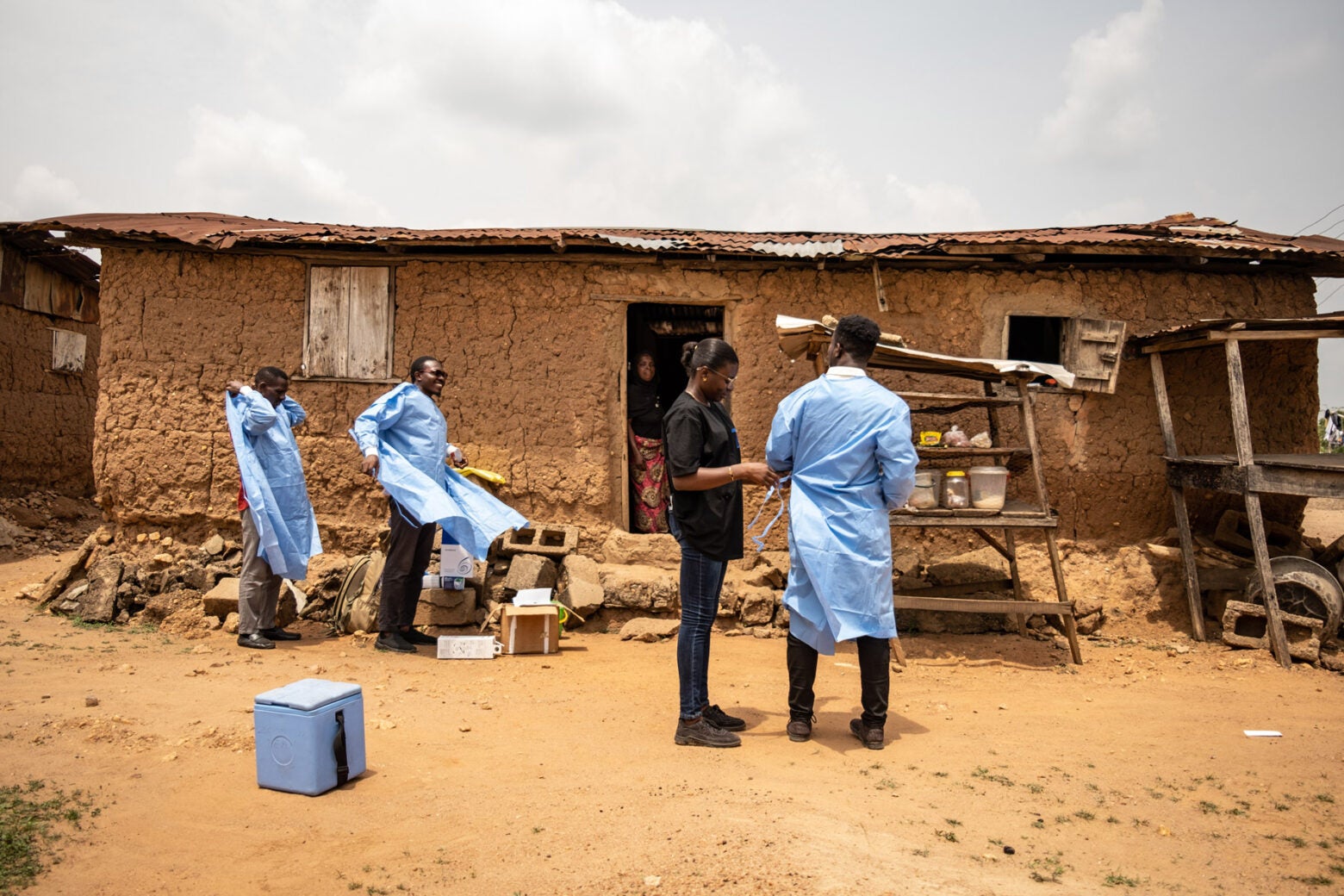
The ACEGID team puts on personal protective equipment before collecting samples from animals at a homestead in Isuada, Nigeria.
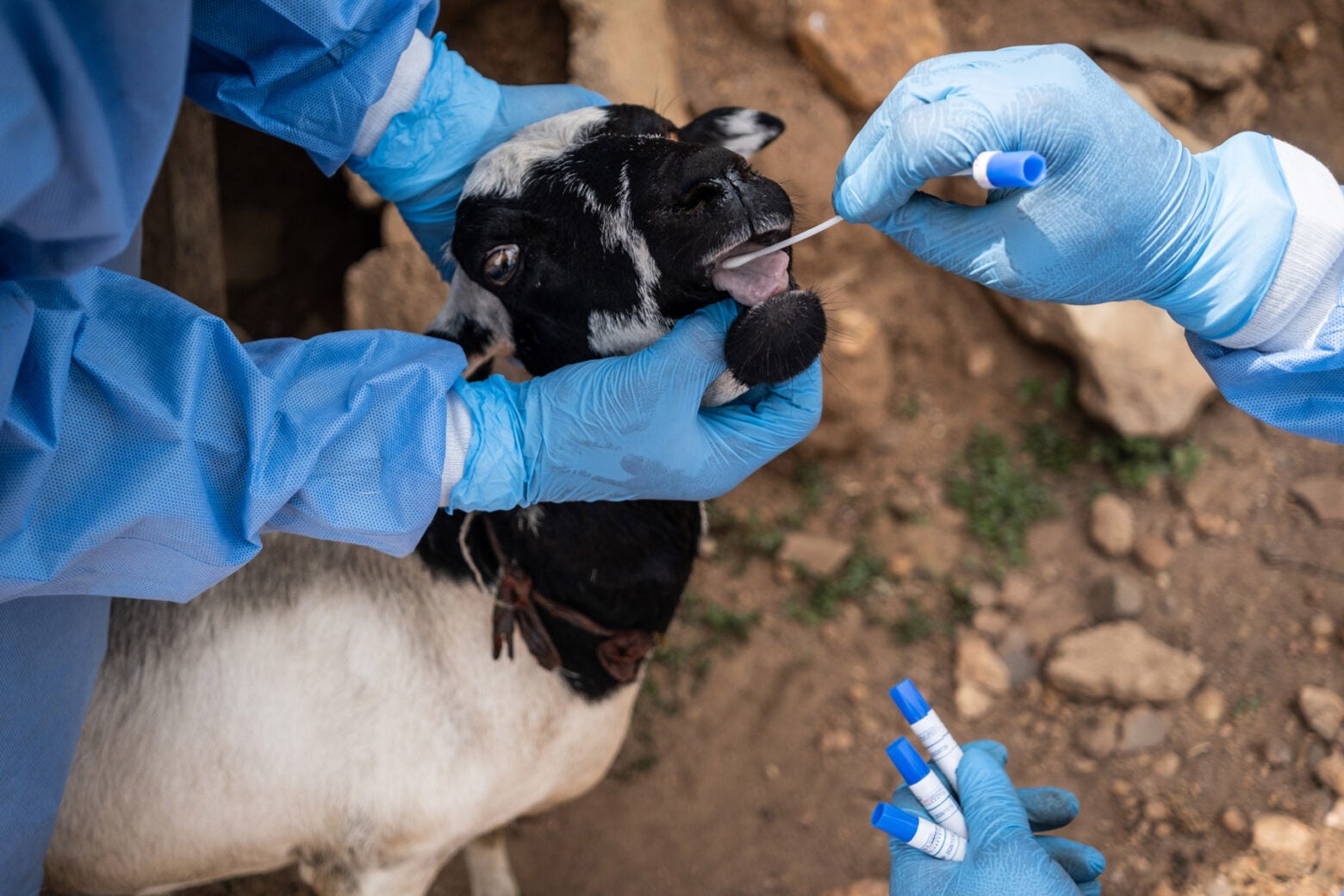
The team collects an oral sample from a goat in Isuada, Nigeria.
It’s another scorching day in March, and I’m watching ACEGID researchers wrestle a goat outside a mud house in a small, dusty community. We’re a three-hour drive from Ede, in Ondo State, a Connecticut-sized territory that has seen 16 deaths from Lassa fever so far this year, making it one of Nigeria’s hotspots for the disease, which is transmitted to humans largely through contact with rodent excretion. Goats contract and also help spread Lassa, which is expected to extend its deadly reach amid climate change.
Lassa fever killed Olesunkanmi Eniayewu, a 40-year-old cocoa farmer in Ondo, in 2019. His father, Oladipo, 75, tells me his son came home from harvesting and then displayed all the hallmark symptoms—a fever, dizziness, aching joints. After 12 days in the hospital being treated with the antiviral drug ribavarin, fluid welled up around his son’s heart, a common Lassa symptom. When doctors operated to remove the fluid, he died.
Oladipo Eniayewu is a frail and elegant bald man who moves about with a cane, his sight severely compromised by cataracts. When we meet, he’s looking dapper in a brightly printed buba and sokoto made from Ankara fabric, but his speech is quiet and reluctant. “He was my firstborn,” he says of his son, “and he was so generous. If I needed money, he’d give it to me. Wherever I wanted to go, he carried me in his car. I never thought he would die before me.”

Oladipo Eniayewu, 75, stands beside the grave of his son, Olesunkanmi, near Owo, Nigeria. Olesunkanmi died of Lassa fever on Christmas day, 2019. He was 40 years old.
Even though Lassa kills far more people than Ebola—about 10,000 die of Lassa each year—it remains sparsely studied. Scientists have sequenced it less than 10,000 times, according to the National Library of Medicine. (COVID, in contrast, has been sequenced over 15 million times.) As such, it’s a priority for ACEGID. In 2021, the center hired the director’s wife, Dr. Anise Happi, a former Harvard postdoc and University of Ibadan professor, to head up zoonotic research on Lassa. Her teams catch and dissect rats, both in Ondo and in Ebonyi, and the genetic material from Lassa-bearing animals figures in CARMEN tests: It’s the control against which blood samples are compared for Lassa infection.
Her teams are also taking blood samples from rodents—one study found that 50 percent of all rats they studied were Lassa-infected.
Anise Happi is currently working on a vaccine for Lassa, as are about 30 other research teams worldwide. She’ll launch the second phase of human testing in October. Still, as she sees it, Lassa will pervade Nigeria for a long time coming. “It’s not just in rats and humans,” she says. “Our research has shown that it’s also in lizards. It’s in goats. It’s in monkeys. If it just mutates a little bit more, it could become inhalatory. It’s not a disease you can easily control.”
In Ondo, many people tell me that pharmacists are insufficiently educated on Lassa; they often cause Lassa victims harm by giving them drugs used for malaria and typhoid fever. Meanwhile, Ondo’s myriad Pentecostal churches frequently champion prayer as the best treatment for disease. One morning, I meet 56-year-old Alaba Aladesanwa, and she tells me that in 2018 when she was delirious with Lassa, she went directly to her church and then lay there, sleeping and hallucinating, for five days as her fellow congregants prayed over her. “I thought I was going to die,” she says, “so I got a ride to the hospital on a motorcycle. Then my pastor called and asked me to come back to the church.” Aladesanwa refused him. She stayed in the hospital and recovered.
Her experience is not unusual, says Michael Olonite. “We Christians have a hard time accepting that science is the best course. I think Muslims are better at it,” he tells me while we’re sitting under a giant shady tree in the village of Ogbese. Olonite is a Catholic catechist who nearly died from Lassa in 2019. While in the hospital he met a fellow Lassa patient, a Muslim imam named Jimoh Tajudeen. The two men are now friends, and they work as Ogbese’s liaisons for ACEGID. When the center’s scientists need to approach, say, a local farmer, to test his goats for Lassa, they make introductions.
Tajudeen stops by to linger with us under the tree. He tells me how, when he contracted Lassa, “My whole body was aching. I was vomiting blood. The local pharmacist prescribed medicine for typhoid fever. This happens all the time.”
Eventually, the two clerics climb onto Olonite’s motorcycle to ride off to the local market, to talk to their neighbors about Lassa and its threats.

Alaba Aladesanwa, 56, had Lassa fever in 2018. She spent days at her church in Owo, receiving prayers for healing but ended up in the hospital where she recovered.

Michael Olonite, a Catholic catechist, and Jimoh Tajudeen, a Muslim imam, became friends during their hospital stay for Lassa fever. They now work as liaisons for ACEGID in Ogbese, Nigeria.
Happi arrives at ACEGID one March morning to find the building dark. He stalks out to the power station, where, amid 46 solar inverters (“the biggest array in Nigeria,” he claims), an ACEGID worker is cradling a burnt electrical cord in his hands, puzzling over what caused a minor power surge. “The system is still working,” he assures Happi. The tech explains they’ve shut it down as a precaution, until they figure out what torched the cord.
Happi is unimpressed. “If the system is working,” he asks the guy, “then why don’t we have power?” The testing equipment needs constant power, and in West Africa brownouts can happen hourly. That’s caused blood samples, which are optimally refrigerated at -80 degrees Celsius, to spoil. It’s also induced Happi to install solar panels at his remote clinics.
The director has a reputation for being a hard driver. “He’s, uh, very demanding,” says Philomena Eromon, his lab manager and former graduate student, laughing in a way at once affectionate and overwhelmed. Still, she seems to adore her boss. She is forever bringing him cold bottles of water. “Hydrate,” she says. “We need you.”
Later that day inside the lab, two scientists are staring at a $110,000 machine called a Fluidigm C1, whose screen bears the message “System Verification in Process.” The scientists are poised to insert a CARMEN panel carrying 96 samples into the machine, which will then look for genetic matches for eight separate pathogens, but the machine had to be shut down because of a power issue. It takes more than 10 minutes to come back online. Then the scientists find the lab has run out of some reagents needed for the tests. It will take a month for Broad to ship them and two months more for the institute to check a previous CARMEN test. The March test I witnessed finally wrapped up in May.
ACEGID has excellent technical equipment, but servicing it creates challenges not uncommon in Africa. Eromon, the lab manager, has a sample preparation machine that needs a new motor. She paid $30,000, up front, for the part late last year. Seven months later, the part had arrived but she was still waiting for a technician to come from South Africa to install it. A prominent African genomicist cautions against reading too much into ACEGID’s logistical challenges. “ACEGID is one of the best research groups I know in Africa,” says Tulio de Oliveira, the director of the South Africa-based Centre for Epidemic Response and Innovation. De Olivera and his team identified the Beta and Omicron variants of COVID.
Like Happi, de Oliveira is tired of questions about how science in Africa compares to the rest of the world. “Look, if you want to critique African science, you need to break things down,” he says. “We probably are not going to send a rocket into outer space, but when it comes to epidemics–no one knows more about them than we do. We’ve always had Ebola. We’ve always had Lassa fever. Add that with the latest technology and, yes, we can become leaders.”

Ayotunde Sijuwola and Ayinla Akeemat Opeyemi, zoonotic research associates at ACEGID, add nucleic acid extracts to a sample in the testing process.

A lab worker loads a Biomark chip onto a reading instrument.
Some of the challenges come from pushing something new. “There’s a lot of logistical issues,” says Elyse Stachler, the lead staff scientist on the CARMEN team at the Broad Institute in Cambridge, Massachusetts. “There’s also a lot of procurement issues getting the reagents needed to run samples. It’s not always easy to get them shipped to Nigeria. And so we’re now just finally starting to build up that capacity.”
CRISPR itself only surged into the headlines in 2020, when the discoverers of the CRISPR “scissors” (a Cas9 protein) won the Nobel Prize in Chemistry. Because scientists can use it to remove, insert, and also find genetic material, it’s frequently heralded as a potential “miracle fix” to life-threatening diseases. At the March 2023 International Summit on Human Genome Editing, in London, an American woman named Victoria Gray proclaimed that experimental CRISPR therapy had zapped her sickle cell disease and enabled her to “dream again without limitations.” Last December, the FDA approved two gene therapies for sickle cell and there’s now rising hope that gene therapy could also liberate patients afflicted with cystic fibrosis, hemophilia, and other illnesses.
But new technologies always face obstacles. The treatment that helped Gray is priced at $2.2 million, not counting the cost of hospital stays, and the price will likely be high for other diseases as well. CRISPR-based monitoring of diseases—what Happi is doing—will require high upfront investments but once in place could benefit multitudes at a low cost-per-person. Proponents see it as a powerful alternative to standard polymerase chain reaction (PCR) testing because it could enable researchers to test scores of samples in a single run. Last year, an article in the American Journal of Public Health noted that it could be especially helpful to “low-income minority communities, which often suffer disproportionate rates of infectious diseases,” in part because “these viruses are challenging and expensive to track.”
But CRISPR-based testing for COVID-19 is not yet commercially viable, despite optimistic predictions at the start of the pandemic. “It’s not clear to me how much sense it makes to use CRISPR. Why not use just the PCR?” asks Elena Ivanova Reipold, the technology innovation lead at FIND, a Swiss NGO working to expand access to reliable diagnostics. She says even though PCR is relatively inexpensive, low- and middle-income countries often struggle with logistical challenges when transporting blood samples. “What’s the point of having technology,” she asks, “if you can’t respond to an outbreak on the periphery of the country?”
Andrew Subica, the lead author on the American Journal of Public Health article, sees things differently. “Nigeria and Sierra Leone are where you need to be applying surveillance technologies the most,” says Subica, an associate professor of social medicine, population, and public health at University of California, Riverside. He adds that “unless someone figures out a way to make these technologies work in a given low-income country, they will never be applied there, and low-income areas will never get any kind of benefit from cutting-edge technologies.”
Subica sees Happi’s project as a golden opportunity. “It’s surprisingly rare,” he says, “that you have a new technology and you get to test it where all these issues need to be worked out.”
Disease science is a suspenseful process, its experiments often fraught with risk. Christian Happi has, quite possibly, more bravery than anyone else in genomics—not many scientists would have done what he did to sequence Ebola. But as he embarks on his work with CRISPR, he’s in the deep of the night all over again. And we’re just going to have to wait until dawn to see how it works out.
Adeleye Roseline Funke provided translation from Yoruba to English.
Top image: Christian Happi enters a lab at ACEGID in Ede, Nigeria in March.




